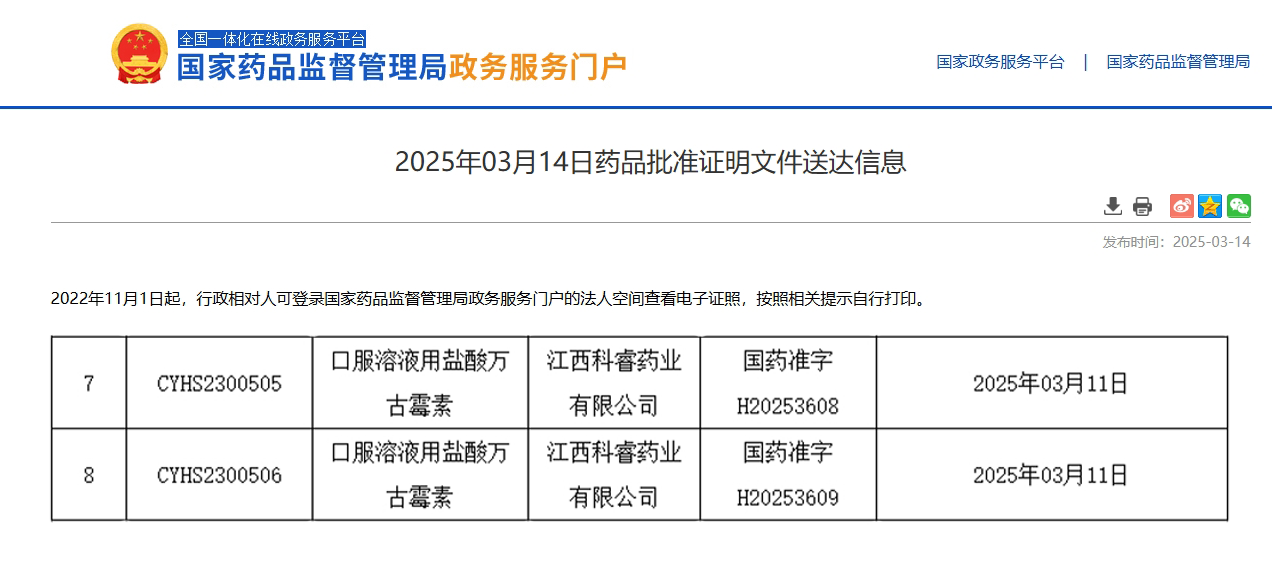
On March 11, Qingfeng Pharmaceutical’s subsidiary, Kvvit Pharmaceuticals, received official approval from China’s National Medical Products Administration (NMPA) for Vancomycin Hydrochloride for Oral Solution, becoming the first company in China to obtain authorization for this product.
New Breakthrough in Targeted Local Therapy
Thanks to its unique pharmacological properties and ease of administration, the oral vancomycin hydrochloride solution offers an efficient and safe treatment option for patients with intestinal infections caused by drug-resistant bacteria, particularly pediatric patients. Its precise local action combined with convenient oral dosing not only improves patients’ quality of life, but also provides a superior clinical choice for treatment strategies.
Clinical Advantages of Oral Formulation
Vancomycin hydrochloride is a glycopeptide antibiotic that has been a cornerstone in combating Gram-positive bacterial infections since its discovery in the 1950s. It plays an irreplaceable role in treating methicillin-resistant Staphylococcus aureus (MRSA) and Clostridioides difficile infections, earning its reputation as the “last line of defense” for humanity. Compared with intravenous administration, , Vancomycin Hydrochloride for Oral Solution offers several clinical advantages. Its oral bioavailability is extremely low (<5%), meaning the drug is barely absorbed systemically and acts directly on infection sites caused by pathogens such as Clostridioides difficile. This avoids risks associated with intravenous administration, including nephrotoxicity, ototoxicity, and “red man syndrome,” making it especially suitable for patients with impaired liver or kidney function.
Expanding the Anti-Infection Portfolio
The development of anti-infectiion drugs is one of Qingfeng Pharmaceutical’s key fields of focus. In recent years, the company has successfully launched a wide range of anti-infection drugs. The approval of the Vancomycin Hydrochloride for Oral Solution further enriches Qingfeng Pharmaceutical’s anti-infection product portfolio, providing patients with new treatment options and a more comprehensive solution.
 Author:Qingfeng Pharmaceutical Group
Author:Qingfeng Pharmaceutical Group
 Posted Time:2025-03-26 00:00
Posted Time:2025-03-26 00:00





